Life history
The total development time ranged from 33 days at 14°C to 19 days at 26°C. The total fecundity ranged from 8.2 to 168.4 eggs per female and it was influenced by fluctuations in temperature and photoperiod (Ekesi et al 1999b; Gitonga et al. 2002).
Host plants
Mainly fabaceous crops.
Crops: African eggplant, African nightshade, African spiderplant, alfalfa, amaranth, avocado, banana, beans (broad bean, common bean, cowpea, French bean, green gram, hyacinth bean, Lima bean, red gram, soybean), capsicum, cashew, cassava, chillies, coffee, cotton, Ficus benjamina, groundnut, kale, lemon, lemon grass, maize, mango, Morus alba, Napier grass, okra, onion, orange, potato, pumpkin, sorghum, spinach, sunflower, sweet potato, tomato, watermelon.
Weeds: Acanthospermum hispidum, Bidens pilosa, Crotalaria bravedens, Crotalaria desertii, Crotalaria ochroleuca, Crotalaria sp., Datura suaveolens, Lactuca inermis, Lantana camara, Leonotis nepetifolia, Melia azadirach, Nycandra physalodes, Polygonum pulcherum, Schkuhria pinnata, Sesbania sesban, Senna didymobotrya, Senna longiracemosa, Sida acuata, Solanum incanum, Sonchus oleracea, Tagetes minuta, Tephrosia villosa ssp. eherenbergiana, Tithonia diversifolia, Triumfetta flavescens, Vernonia hochstetteri.
Vector capacity
None identified, but possible mechanical distribution of phytopathogenic fungi and bacteria.
Damage and symptoms
Considered to be a serious pest of cowpeas (Vigna unguiculata) throughout tropical Africa, feeding in the flowers on pollen and other floral tissues and causing flowers to fall and crop yields to be reduced. Leaf buds and bracts / stipules of cowpea are damaged during the pre-flowering period, resulting in browning/drying of stipules, leaf or flower buds, deformation of leaf buds and bracts/stipules with a brownish-yellow mottled appearence (Jackai & Singh 1988; Gahukar 2004).
Detection and control strategies
Detection: Megalurothrips sjostedti are significantly more attracted to blue sticky trap as compared to yellow coloured traps. Efficacy of the blue sticky traps for detection of Megalurothrips sjostedti were found to be enhanced in the presence of kairomonal attractant LUREM-TR (Nielsen et al. 2010; Muvea 2011).
Cultural control: The most effective cultivation method is the intercropping of cowpea or beans with cereal crops such as pearl millet (Gahukar 2004), sorghum or maize (Dissemond & Hindorf 1990; Kyamanywa & Tukahirwa 1988; Ekesi et al. 1999a; Ngakou et al. 2008).
Host plant resistance: High yield cowpea varieties such as TVx3236 have shown low to moderate level of resistance against Megalurothrips sjostedti (Jackai & Daoust 1986; Salifu et al. 1988). Improved cowpea varieties like IT90K-76, IT90K-59, and IT90K 277-2 are resistant against thrips, aphids and bruchids (Singh et al. 2002).
Biological control: Major predators are the pirate bugs Orius amnesius and O. albidipennis - Anthocoridae (Matteson 1982). Biocontrol based pest management strategy for Megalurothrips sjostedti on cowpea indicated Cerenisus femoratus to be an effective parasitoid in Cameroon. Release of this parasitoids in Benin and Ghana resulted in increase of parasitism rates to upto 60%, indicating its potential to be an effective biological control agent (Tamo et al. 2002). Application of entomopathogenic fungi, Metarhizium anisopliae was effective for management of Megalurothrips sjostedti on cowpea (Ekesi et al. 1998; Ekesi & Maniania 2002; Tamo et al. 2003; Ngakou et al. 2008).
Botanical pesticides: Application of Neem Seed Kernal Extract at 20% was as effective as three applications of cypermethrin for management of Bean thrips (Saxena & Kidiavai, 1997).
Additional notes
-
Biogeography
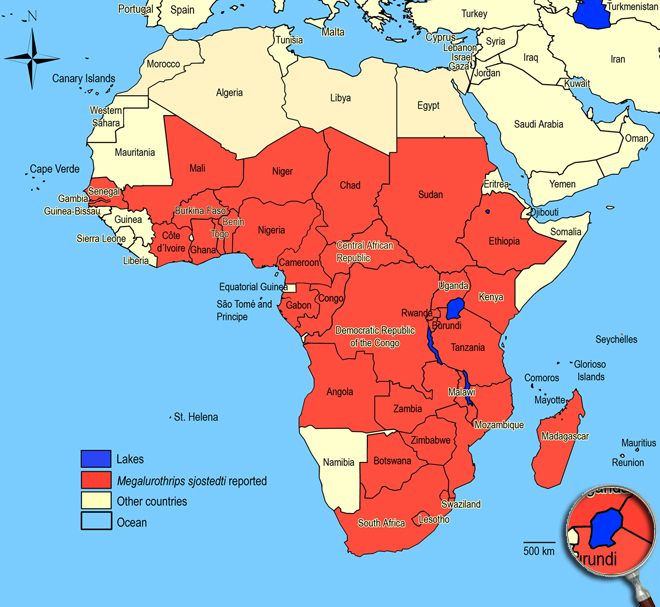
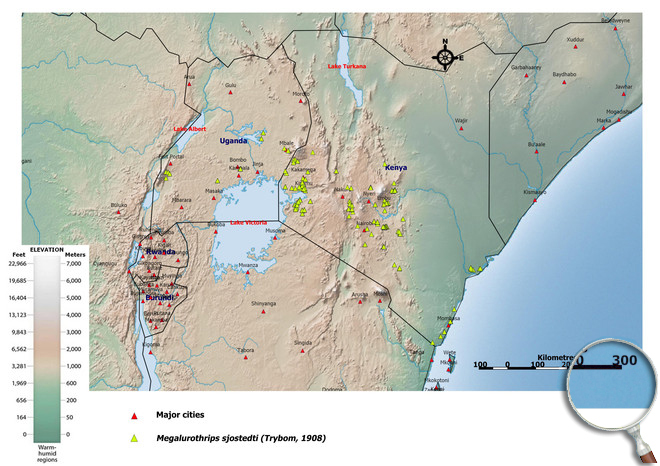
Afrotropical Region, Yemen. Angola (Lund forest of Luachimo, forest of Governor's Palace Vila Luso, Lunda - Dundo),
Burundi (Commune of Gihanga),
Cameroon,
Central African Republic,
Cape Verde Islands (Santo Antão - Ribeira de Paul),
Chad,
Congo (Brazzaville),
Gabon,
Ghana,
Gambia (Yundum, Kombo, Abuko, Brikama),
Ivory Coast,
Kenya, Mozambique (Catembe),
Niger (Niamey, Diadia),
Nigeria,
Senegal,
Seychelles,
South Africa (in all parts),
Tanzania (Kibonoto, Kilimanjaro - 1300 m),
Togo (Wonugba, Dadja),
Uganda (Kampala),
Zimbabwe.
Afun JVK, Jackai LEN & Hodgson CJ (1991). Calendar and monitored insecticide application for the control of cowpea pests. Crop Protection. 10 (5): 363-370
Akingbohungbe AE (1982). Seasonal variation in cowpea crop performance at Ife-Ife, Nigeria, and the relationship to insect damage. Insect Science and its Application. 3: 287-296
Alabi OY, Odebiyi JA & Jackai LEN (2003). Field evaluation of cowpea cultivars (Vigna unguiculata [L.] Walp.) for resistance to flower bud thrips (Megalurothrips sjostedti Trybom) (Thysanoptera: Thripidae). International Journal of Pest Management. 49 (4): 287-291
Alabi OY, Odebiyi JA & Tamo M (2004). Effect of host plant resistance in some cowpea (Vigna unguiculata (L.) Walp.) cultivars on growth and developmental parameters of the flower bud thrips, Megalurothrips sjostedti (Trybom). Crop Protection. 23 (2): 83-88
Alghali AM (1992). On-farm evaluation of control strategies for insect pests in cowpea with emphasis on flower thrips, Megalurothrips sjostedti Trybom (Thysanoptera, Thripidae). Tropical Pest Management. 38 (4): 420-424
Amatobi CI (1994). Field-evaluation of some insecticides for the control of insect pests of cowpea (Vigna unguiculata (L.) Walp.) in the Sudan savanna of Nigeria. International Journal of Pest Management. 40 (1): 13-17
Ampong-Nyarko K, Reddy KVS, Nyang'or RA & Saxena KN (1994). Reduction of insect pest attack on sorghum and cowpea by intercropping. Entomologia Experimentalis et Applicata. 70 (2): 179-184
Asiwe JAN, Nokoe S, Jackai LEN & Ewete FK (2005). Does varying cowpea spacing provide better protection against cowpea pests? Crop Protection. 24 (5): 465-471
Atachi P & Ahohuendo BC (1989). Comparison of some parameters characteristic of the population dynamics of Megalurothrips sjostedti (Trybom) and Maruca testulalis (Geyer) on a single plant host (Vigna). Insect Science and its Application. 10 (2): 187-197
Atachi P & Dannon EA (1999). Comparative population dynamics of Maruca vitrata (Fabricius) (Lepidoptera, Pyralidae) and Megalurothrips sjostedti (Trybom) (Thysanoptera, Thripidae) defined by assessing of flowers infestations and onset probabilities in different patterns of cowpea-pigeon pea intercropping in South Benin. Bulletin de la Société Zoologique de France. 124 (3): 239-260
Atachi P & Sourokou B (1992). Effects of Decis® and Systoate® on Megalurothrips sjostedti (Trybom) in cowpea. Insect Science and its Application. 13 (2): 279-286
Bagnall RS (1913). Brief descriptions of new Thysanoptera - I. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 8) 12: 290-299
Bal AB (1991). Action threshold for flower thrips on cowpea (Vigna unguiculata (L.) Walp.) in Senegal. Tropical Pest Management. 37 (4): 363-367
Bhatti JS (1969). The taxonomic status of Megalurothrips Bagnall (Thysanoptera: Thripidae). Oriental Insects. 3 (3): 239-244
Bottenberg H, Tamò M & Singh BB (1998). Occurrence of phytophagous insects on wild Vigna sp. and cultivated cowpea: comparing the relative importance of host-plant resistance and millet intercropping. Agriculture, Ecosystems & Environment. 70 (2-3): 217-229
Bournier A (1970). Thysanoptères du Gabon. Extrait de la Revue Biologia Gabonica. 6 (2): 151-168
Bournier A (1979). Thysanoptères ďAngola. VII. Garcia de Orta, Série de Zoologia. 8 (1-2): 1-10
Daiber KC (1994). Injurious insects, spider-mites and nematodes on peas and green beans in southern Africa. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz. 101 (1): 99-107
Diambo B, Yagoua D, Renou A & Martin T (1993). New prospects for the protection of cowpea crops in Chad. Sahel PV Info. 58: 13-21
Dissemond A & Hindorf H (1990). Influence of sorghu/maize/cowpea intercropping on the insect situation at Mbita/Kenya. Journal of Applied Entomology. 109: 144-150
Ehlers JD & Hall AE (1997). Cowpea (Vigna unguiculata L. Walp). Field Crops Research. 53 (1-3): 187-204
Ekesi S & Maniania NK (2000). Susceptibility of Megalurothrips sjostedti developmental stages to Metarhizium anisopliae and the effects of infection on feeding, adult fecundity, egg fertility and longevity. Entomologia Experimentalis et Applicata. 94 (3): 229-236
Ekesi S & Maniania NK (2002). Metarhizium anisopliae: An effective biological control agent for the management of thrips in horti- and floricultural crops in africa. In: Upadhyay, R.K. (sd.) Advances in Microbial Control of Insect Pests. Kluwer Academic/Plenum Publishers, The Netherlands, pp. 165-180
Ekesi S, Maniania NK, Ampong-Nyarko K & Onu I (1998). Potential of the entomopathogenic fungus, Metarhizium anisopliae (Metsch.) Sorokin for control of the legume flower thrips, Megalurothrips sjostedti (Trybom) on cowpea in Kenya. Crop Protection. 17 (8): 661-668
Ekesi S, Maniania NK, Ampong-Nyarko K & Onu I (1999). Effect of intercropping cowpea with maize on the performance of Metarhizium anisopliae against Megalurothrips sjostedti (Thysanoptera: Thripidae) and predators. Environmental Entomology. 28 (6): 1154-1161
Ekesi S, Maniania NK & Lwande W (2000). Susceptibility of the legume flower thrips to Metarhizium anisopliae on different varieties of cowpea. BioControl. 45 (1): 79-95
Ekesi S, Maniania NK & Onu I (1999). Effects of temperature and photoperiod on development and oviposition of the legume flower thrips, Megalurothrips sjostedti. Entomologia Experimentalis et Applicata. 93 (2): 149-155
Ekesi S, Maniania NK, Onu I & Lohr B (1998). Pathogenicity of entomopathogenic fungi (Hyphomycetes) to the legume flower thrips, Megalurothrips sjostedti (Trybom) (Thysan., Thripidae). Journal of Applied Entomology. 122 (9-10): 629-634
Faure JC (1960). Thysanoptera of Africa - 3. Journal of the Entomological Society of Southern Africa. 23 (1): 16-44
Fritsche ME & Tamò M (2000). Influence of thrips prey species on the life-history and behaviour of Orius albidipennis. Entomologia Experimentalis et Applicata. 96 (2): 111-118
Gahukar RT (2004). Bionomics and management of major thrips species on agricultural crops in Africa. Outlook on Agriculture. 33 (3): 191-199
Gitonga LM, Lohr B, Overholt WA, Magambo JK & Mueke JM (2002a). Temperature-dependent development of Megalurothrips sjostedti and Frankliniella occidentalis (Thysanoptera: Thripidae). African Entomology. 10 (2): 325-331
Gitonga LM, Overholt WA, Lohr B, Magambo JK & Mueke JM (2002b). Functional response of Orius albidipennis (Hemiptera: Anthocoridae) to Megalurothrips sjostedti (Thysanoptera: Thripidae). Biological Control. 24 (1): 1-6
Hill D (1983). Agricultural insect pests of the tropics and their control, (2nd edition). Cambridge University Press, Cambridge, 746 pp
Ingram WR (1969). Observations on the pest status of bean flower thrips in Uganda. East African Agricultural and Forestry Journal. 34: 482-484
Jackai LEN & Singh SR (1988). Screening Techniques for Host Plant Resistance to Insect Pests of cowpea. Tropical Grain Legume Bulletin 35: 2-18
Jackai LEN & Daoust RA (1986). Insect pests of cowpeas. Annual Review of Entomology. 31: 95-119
Karny H (1912). Revision der von Serville aufgestellten Thysanopteren-Genera. Zoologische Annalen. 4 (4): 322-344
Karny H, van Leeuwen-Reijnvaan W & van Leeuwen-Reijnvaan J (1914). Beiträge zur Kenntnis der Gallen von Java. Zweite Mitteilung über die javanischen Thysanopterocecidien und deren Bewohner. Zeitschrift für Wissenschaftliche Insektenbiologie. 10: 355-369
Kyamanywa S, Baliddawa CW & Ampofo KJO (1993). Effect of maize plants on colonization of cowpea plants by bean flower thrips, Megalurothrips sjostedti. Entomologia Experimentalis et Applicata. 69 (1): 61-68
Kyamanywa S, Baliddawa CW & Omolo E (1993). Influence of cowpea/maize mixture on generalists predators and their effect on population density of the legume flower thrips, Megalurothrips sjostedti Trybom (Thysanoptera, Thripidae). Insect Science and its Application. 14 (4): 493-499
Kyamanywa S & Tukahirwa EM (1988). Effect of mixed cropping beans, cowpeas and maize on population densities of bean flower thrips, Megalurothrips sjostedti (Trybom) (Thripidae). Insect Science and its Application. 9 (2): 255-259
Lewis T (1973). Thrips: Their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp
Lewis T (1997). Thrips as crop pests. CAB International, Wallingford, 740 pp
Matteson PC (1982). The effect of intercropping with cereals and minimal permethrin application on insect pests of cowpea and their natural enemies in Nigeria. Tropical Pest Management. 28 (4): 372-380
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Moulton D (1936). Thysanoptera from Africa. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 10) 17: 493-509
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International,Wallingford and New York, 70 pp
Muvea AM (2011). The Potential of Coloured Sticky Traps with Kairomonal Attractants
(LUREM-TR) in Management of Thrips on Tomato and French beans. Unpublished thesis, Jomo Kenyatta University of
Agriculture and Technology, Nairobi, Kenya, 107 pp
Nabirye J, Nampala P, Kyamanywa S, Ogenga-Latigo MW, Wilson H & Adipala E (2003). Determination of damage-yield loss relationships and economic injury levels of flower thrips on cowpea in eastern Uganda. Crop Protection. 22 (7): 911-915
Nderitu JH, Kasina MJ, Nyamasyo GN, Waturu CN & Aura J (2008). Management of thrips (Thysanoptera: Thripidae) on French beans (Fabaceae) in Kenya: Economics of insecticide applications. Journal of Entomology. 5 (3): 148-155
Nielsen M-C, Worner S, Chapman B, de Kogel W-J, Perry N, Sansom C, Murai T, Muvea A., Subramanian S, Davidson M & Teulon D. (2010). Optimising the use of allelochemicals for thrips pest management. Proceedings of the International Society of Chemical Ecology Conference, 26th Annual Meeting, July 31st-August 4th 2010, Tours, France. 324 pp
Ngakou A, Tamò M, Parh IA, Nwaga D, Ntonifor NN, Korie S & Nebane CLN (2008). Management of cowpea flower thrips, Megalurothrips sjostedti (Thysanoptera, Thripidae), in Cameroon. Crop Protection. 27 (3-5): 481-488
Nyiira ZM (1971). The status of insect pests of cowpeas (Vigna unguiculata (L.) Walp.) in Uganda and their control. Pest Articles and News Summaries. 17 (2): 194-197
Ofuya TI (1991). Observations on insect infestation and damage in cowpea (Vigna unguiculata) intercropped with tomato (Lycopersicon esculentum) in a rain-forest area of Nigeria. Experimental Agriculture. 27 (4): 407-412
Okwakpam BA & Youdeowei A (1980). The annotated key to four species of thrips (Thysanoptera) attacking edible legumes in Nigeria. Bulletin de l´Institut Fondamental d´Afrique Noire, Série A, Sciences Naturelles. 42: 157-165
Oparaeke AM, Dike MC & Amatobi CI (2006). Field activity of three mixture levels of plant extract formulations for the management of post-flowering insect pests of cowpea, Vigna unguiculata (L.) Walp. - The flower thrips, Megalurothrips sjostedti (Trybom). Journal of Sustainable Agriculture. 28 (4): 45-54
Oparaeke AM, Dike MC & Amatobi CI (2006). Botanical pesticide mixtures for insect pest management on cowpea, Vigna unguiculata (L.) Walp. plants - The legume flower bud thrips, Megalurothrips sjostedti Trybom. Journal of Sustainable Agriculture. 29 (1): 5-13
Palmer JM (1987). Megalurothrips in the flowers of tropical legumes: a morphometric study, pp. 480-495. In Holman J, Pelikan J, Dixon AFG & Weismann L [eds.], Population structure, genetics and taxonomy of aphids and Thysanoptera. SPB Academic Publishing, The Hague, 542 pp
Palmer JM (1990). Identification of the common thrips of Tropical Africa (Thysanoptera, Insecta). Tropical Pest Management. 36 (1): 27-49
Palmer JM, Mound LA & du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Pitkin BR & Mound LA (1973). A catalogue of West African Thysanoptera. Bulletin de ľInstitut Fondamental ďAfrique Noire, Série A. 35 (2): 407-449
Priesner H (1952). On some Central African Thysanoptera. Bulletin de ľInstitut Fondamental de ľAfrique Noire. 14 (3): 842-880
Salifu AB & Hodgson CJ (1987). Dispersion patterns and sequential sampling plans for Megalurothrips sjostedti (Trybom) (Thysanoptera, Thripidae) in cowpeas. Bulletin of Entomological Research. 77 (3): 441-449
Salifu AB, Hodgson CJ & Singh SR (1988). Mechanism of resistance in cowpea (Vigna unguiculata (L.) Walp.) genotype, Tvx-3236, to the bean flower thrips, Megalurothrips sjostedti (Trybom) (Thysanoptera, Thripidae). 1. Ovipositional nonpreference. Tropical Pest Management. 34 (2): 180-184
Salifu AB & Singh SR (1987). Evaluation of sampling methods for Megalurothrips sjostedti (Trybom) (Thysanoptera, Thripidae) on cowpea. Bulletin of Entomological Research. 77 (3): 451-456
Salifu AB, Singh SR & Hodgson CJ (1988). Mechanism of resistance in cowpea (Vigna unguiculata (L.) Walp.) genotype, Tvx-3236, to the bean flower thrips, Megalurothrips sjostedti (Trybom) (Thysanoptera, Thripidae). 2. Nonpreference and antibiosis. Tropical Pest Management. 34 (2): 185-188
Saxena RC & Kidiavai EL (1997). Neem seed extract spray applications as low-cost inputs for management of the flower thrips in the cowpea crop. Phytoparasitica. 25 (2): 99-110
Sing SR, van Emden HF & Taylor TA (1978). Pests of grain legumes: ecology and control. Academic Press, London, 454 pp.
Singh BB, Ehlers JD, Sharma B & Freire-Filho FR (2002). Recent progress in cowpea breeding. In: Fatokun CA, Tarawali SA, Singh BB, Kormawa PM, Tamo M (eds). Challenges and opportunities for enhancing sustainable cowpea production. Ibadan: IITA, p. 22 - 40
Steinweden JB (1933). Key to all known species of the genus Taeniothrips Amyot & Serville (Thysanoptera: Thripidae). Transactions of the American Entomological Society. 59 (4): 269-293
Tamò M, Baumgärtner J, Delucchi V & Herren HR (1993). Assessment of key factors responsible for the pest status of the bean flower thrips Megalurothrips sjostedti (Thysanoptera, Thripidae) in West Africa. Bulletin of Entomological Research. 83 (2): 251-258
Tamò M, Baumgärtner J & Gutierrez AP (1993). Analysis of the cowpea agroecosystem in West Africa. 2. Modelling the interactions between cowpea and the bean flower thrips Megalurothrips sjostedti (Trybom) (Thysanoptera, Thripidae). Ecological Modelling. 70 (1-2): 89-113
Tamò M, Arodokoun DY, Zenz N, Tindo M, Agboton C & Adeoti R (2002). The importance of alternative host plants for the biological control of two key cowpea insect pests, the pod borer Maruca vitrata (Fabricius) and the flower thrips Megalurothrips sjostedti (Trybom). In: Fatokun CA, Tarawali SA, Singh BB, Kormawa PM, Tamò M (eds). Challenges and opportunities for enhancing sustainable cowpea production. Ibadan: IITA
Tamò M, Ekesi S, Maniania NK & Cherry A (2003). Biological control, a non obvious component of Integrated Pest Management for Cowpea. In: Neuenschwander, C., Borgemeister, C., Langewald, J. (eds.). Biological Control in Integrated Pest Management Systems in Africa. CAB International, Wallingford, pp. 295-309
Tanzubil PB (1991). Control of some insect pests of cowpea (Vigna unguiculuta) with neem (Azadirachta indica A Juss.) in Northern Ghana. Tropical Pest Management. 37 (3): 216-217
Taylor TA (1969). On the population dynamics and flight activity of Taeniothrips sjostedti (Tryb.) (Thysanoptera: Thripidae) on cowpea. Bulletin of Entomological Society of Nigeria. 2: 142-145
Taylor TA (1974). On the population dynamics of Taeniothrips sjostedti (Tryb.) (Thysanoptera: Thripidae) on cowpea and alternate host, Centrosema pubescens Benth. in Nigeria. Revue de Zoologie Africaine. 88: 689-702
Trybom F (1908). Physapoda. Wissentschaftliche Ergebnisse der Schwedischen Zoologischen Expedition nach dem Kilimandjaro, dem Meru, und dem umgebenden Massaisteppen Deutsch-Ostafrikas, 1905-1906. 16: 1-23
zur Strassen R (1958). Notes on Thysanoptera of tropical Africa and St. Helena. Journal of the Entomological Society of Southern Africa. 21 (1): 69-79
zur Strassen R (1960). Catalogue of the known species of South African Thysanoptera. Journal of the Entomological Society of Southern Africa. 23 (2): 321-367
zur Strassen R (1980). Thysanopterologische Notizen (5) (Insecta: Thysanoptera). Senckenbergiana Biologica. 60 (3-4): 191-202
zur Strassen R (1983). Thysanopterologische Notizen (6) (Insecta: Thysanoptera). Senckenbergiana Biologica. 63 (3-4): 191-209
zur Strassen R (2003). Die terebranten Thysanopteren Europas und des Mittelmeer-Gebietes. Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise, 74. Teil. Goecke & Evers, Keltern, Germany, 277 pp